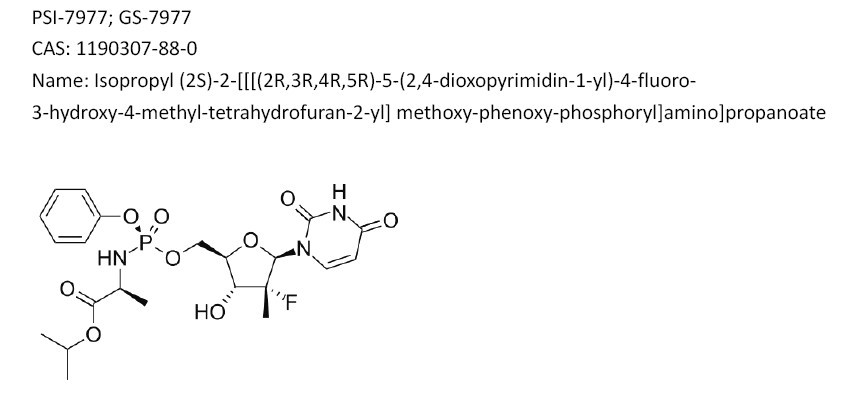
Gilead Sciences announced that the company has submitted a New Drug Application, or NDA, to the FDA for approval of sofosbuvir, a once-daily oral nucleotide analogue for the treatment of chronic hepatitis C virus, or HCV, infection.
The data submitted in this NDA support the use of sofosbuvir and ribavirin, or RBV, as an all-oral therapy for patients with genotype 2 and 3 HCV infection, and for sofosbuvir in combination with RBV and pegylated interferon, or peg-IFN, for treatment-naive patients with genotype 1, 4, 5 and 6 HCV infection. If approved, sofosbuvir would shorten HCV therapy to 12-16 weeks, and depending on the genotype, would either eliminate or reduce the duration of peg-IFN injections.